What Is the Conjugate Base of No3-
A more general definition is that a conjugate base is the base member X- of a pair of compounds that transform into each other by gaining or losing a proton. HCO 3 H H 2 CO 3 Carbonic acid or H2CO3 will be the conjugate acid of.

Is Hno3 An Acid Or Base Strong Vs Weak Nitric Acid
NO3- is the conjugate base of HNO3 which.
. In the BrønstedLowry definition of acids and bases a conjugate acidbase pair consists of two substances that differ only by the presence of a proton H. Since the equilibrium has to maintain the conjugate. Nitrate is a monovalent anion with formula NO3- which is the conjugate base of Nitric acid HNO3.
- In reaction b HNO3 is the acid because it donates a proton to H2O and forms the conjugate base NO3. The hydronium ion H3O is the conjugate acid of the. The conjugate base of any species is that species less a proton.
TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid. An example of an acid-base reaction is the following. The base definition says it is accepting proton to become an acid in reverse reaction.
It donates a hydrogen ion to the water which acts as a base in the forward reaction. The reaction resulting in the conjugate base of HNO3 is HNO3 H2O H3O NO3-. HNO3 H20 H NO3-NO3- is conjugate base.
Since H2O is the proton acceptor it is the base and it forms the conjugate acid. Is NH4 A conjugate base of NH3. To improve on this.
NH2 is the conjugate base of NH3. Who are the experts. The conjugate base of H2 SO4 is HSO4.
Ammonia less a proton is the amide ion NH2. The balanced reaction can be written as. What is the pH of a conjugate base.
Asked Sep 18 2016 in Chemistry by Gibby. We review their content and use your feedback to. Conjugate bases and conjugate acids are formed in acid-base reactions where an actual acid reacts with an actual base.
A conjugate acid is formed when a. Nitrate or NO3- is the conjugate base of HNO3. The conjugate base to HSO4- is SO42-.
H2 SO4 loses a proton H to form the conjugate base. The term conjugate base in the chemical community is typically used in association with the term conjugate acid and comes from the Bronsted-Lowry theory of Acids. Here N H 4 acts as acid and donates a proton.
Let us take the example of bicarbonate ions reacting with water to create carbonic acid and. NO3 is not an acid but would be an acid if it had H hydrogen in front of it To improve on this. NO3- is the conjugate base of HNO3 which is a strong acid.
The acid dissociation constant Ka equals 126 10-2 for HSO4- and. A chemical reaction in which reactants A and B form the product C is studied in the laboratory. Experts are tested by Chegg as specialists in their subject area.
See answer 1 Best Answer. 10 enables us to. Conjugate Acid-Base Pairs Ordered by Strength Acids Bases strong weak HClO 4 ClO 4 H 2SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3O H 2O H 2C 2O 4 oxalic acid HC 2O 4 H 2SO 3.
In most cases the acid molecule. What is h2so4 conjugate base. What is HClO2 conjugate base.
NH3 can donate a proton act as an acid. Indicate the species that are conjugate acids and conjugate bases. A conjugate base contains one less H atom and one more - charge than the acid that formed it.
HSO4- acid NH3. What is the conjugate base of HNO 3. The researcher carries out the reaction with differing relative amounts of reactants and.
HNO3 nitric acid is the Bronsted-Lowry acid. NO3 is not an acid but would be an acid if it had H hydrogen in front of it. ClO2 is the conjugate base of HClO2 and H3O is the conjugate acid of H2O.
Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. N H 4 H 2 O N H 3 H 3 O. Hence water acts as a base and accepts protons.
For the following reactions label each species as an acid or a base.

Acids And Bases Section 19 1 Acid Base Theories Objectives Define The Properties Of Acids And Bases Ppt Download
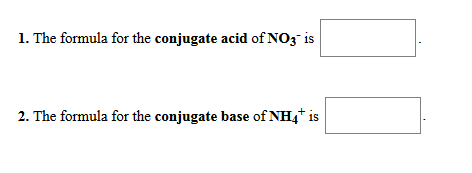
Solved 1 The Formula For The Conjugate Acid Of No3 Is 2 Chegg Com

Solved Identify The Conjugate Acid Or Base Of Each Of The Chegg Com
Comments
Post a Comment